Heron Pharma showcases expertise at CPhI China 2020
On June 17-20th, 2021, the 27th Annual Meeting of Chinese Society of Dermatology (CSD2021), an annual academic event of Chinese dermatology, was grandly held in the scorching summer and in the famous ancient city - Xi'an. With the theme of "academic guidance and clinical consolidation", the conference was held offline and online in combination. Thousands of dermatologists, scientific researchers and representatives of cooperative enterprises gathered in Xi'an for an academic feast. At the same time, nearly 10,000 people participated in the event online.

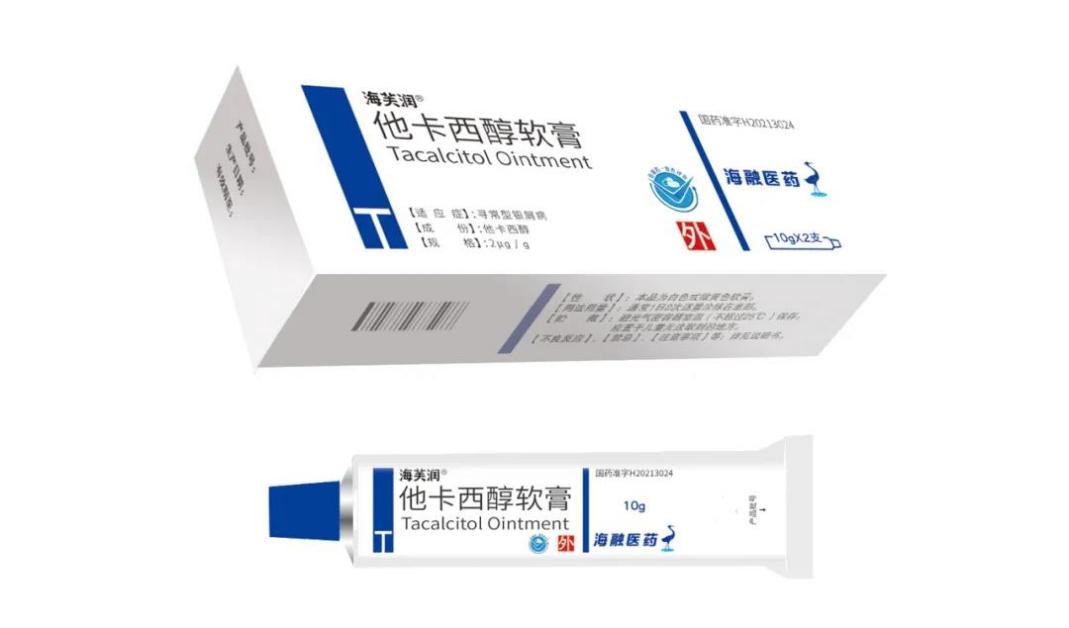
Haifurun® Tacalcitol Ointment of Heron Pharma was approved by the National Medical Products Administration on January 19, 2021 (H20213024). As the first imitation drug, Haifurun® successfully passed the Drug Consistency Evaluation and filled the vacancy of Domestic Tacalcitol Ointment in the past 24 years. Heron Pharma launch this drug and it attracted the attention of many experts and scholars. Heron Pharma will participate in more academic activities and continue to carry out academic and brand promotion.
Post time: Jun-29-2021